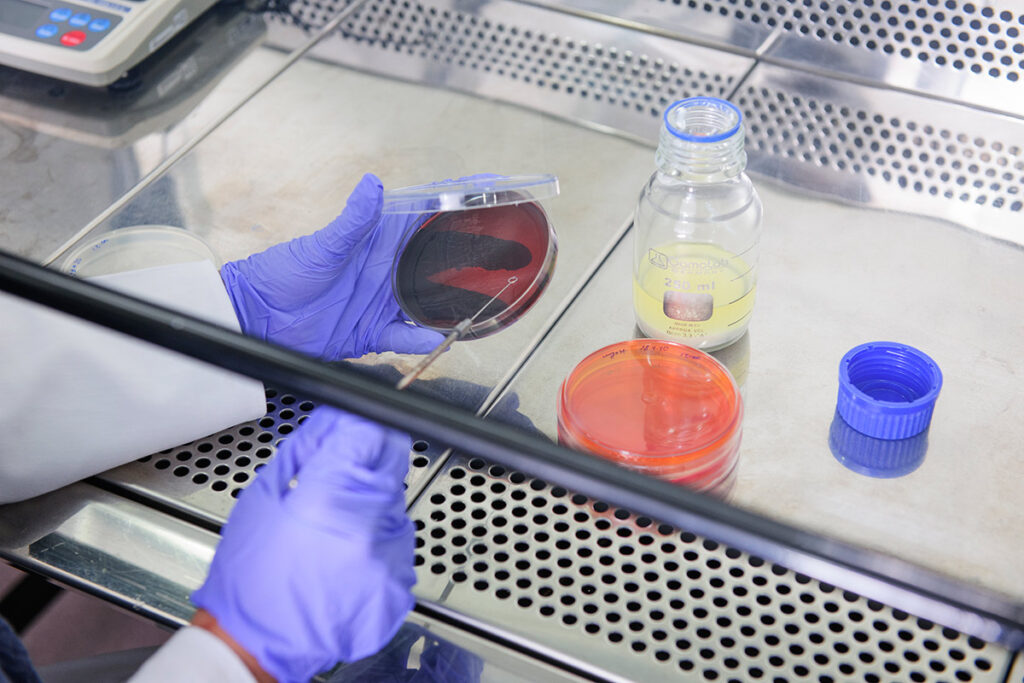
Our Knowledge, Our Quality Assurance
We are proud to announce that the Good Manufacturing Practice (GMP) certification for Behansar’s production lines has been renewed for a new term. This achievement reaffirms our commitment to quality, safety, and compliance with standards in the production of pharmaceutical raw materials.
Drawing on our expertise and experience, we deliver high quality raw materials supported by a diverse product portfolio and specialized technical support. Your trust is our most valuable asset. We also commend the dedication, hard work, and knowledge of all our colleagues who contributed to this success.





- Selection of raw materials: We use high-quality minerals as the main raw materials for production, and we ensure the purity and appropriateness of the raw materials with a variety of tests.
- Process Optimization: We implement efficient and controlled manufacturing processes to maintain consistent quality. We will regularly review and improve production techniques to provide optimal conditions and minimal changes.
- Quality control methods: We establish strong quality control mechanisms to monitor each stage of production so that we can quickly identify and resolve any deviations or quality problems.
- Compliance with standards: compliance with standards and regulations related to production based on GMP (Good manufacturing practice) is our strategy.
- Employee training: By regularly training employees involved in the production process, we educate them about standards, good manufacturing practices, and quality control methods.
- Collaboration with suppliers: We work closely with reliable and reputable suppliers who consistently provide us with high-quality raw materials, and we have developed a comprehensive supplier selection process.
- Continuous Improvement: We have created a culture of continuous improvement. We regularly evaluate production processes, collect customer feedback and implement necessary changes to increase quality and customer satisfaction.